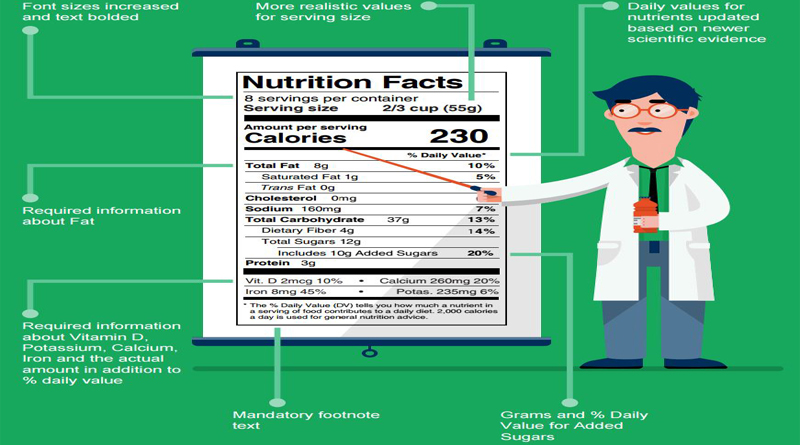
New deadline extended to 2020 for companies with $10 million or more in food sales.
The U.S. Food and Drug Administration has issued a final rule to extend the compliance dates for updating Nutrition Facts and Supplement Facts labels, from July 26, 2018, to January 1, 2020, for manufacturers with $10 million or more in annual food sales. Manufacturers with less than $10 million in annual food sales will receive an extra year to comply—until January 1, 2021. The agency published a proposed rule to extend the compliance date in September 2017, and this rule finalizes that extension.
After considering a range of stakeholder comments, the FDA recognized the need for manufacturers to have additional time to make required changes. The approximately 18-month extension accomplishes this goal, the agency said, and will provide sufficient time to transition to the new version of the Nutrition Facts label.
The FDA also said it’s committed to ensuring that all manufacturers have guidance to help implement the required label changes by the upcoming compliance dates. A full list of Nutrition Facts-related guidance documents is available on the FDA website.
Source: Nutraceuticals World